PFAS removal: The forever chemical now has an expiration date

DOWNLOAD THE PAPER
Abstract
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a group of anthropogenic chemicals. Current information pertains mainly to Perfluorooctane sulfonate (PFOS) and Perfluorooctanoic acid (PFOA), and recently perfluorohexane sulfonate (PFHxS), but there are thousands of various PFAS compounds with different American Chemical Society Chemical Abstracts Service (CAS) Registry Numbers.
These chemicals are persistent in the environment and humans. They have a negative effect on the health of those individuals exposed to them. In response to the concerns, certain guidelines have been put in place globally, so it is clear what levels of PFAS should exist in water used for specific purposes.
Many treatment methods appeared based on the characteristics of PFAS. The most common way to treat PFAS contaminated water is the use of granular activated carbon (GAC) and/or ion exchange (IX) resins. However, problems arise in terms of efficiency.
A new absorbent media of MyCelx filtering and polishing has been developed by OLEOLOGY and has been approved (through in-field operations) and accepted Australia wide. The treatment system, utilising MyCelx technology, has lower cost, requires a smaller footprint, and has proven capability to achieve below level of detection for all PFAS and precursors. The National Environmental Management Plan on PFAS (PFAS NEMP 3.0) is to be released soon and indications are more PFAS analytes have been added.
OLEOLOGY water treatment systems infused with MyCelx technologies have been deployed and the results tested for eight new analytes including GenX and ADONA.
Introduction
What is a PFAS (The ‘Forever Chemical’)?
Perfluoroalkyl substances (PFAS) are a group of anthropogenic organofluorine compounds, a class of highly fluorinated substances. Also referred to as fluorochemicals, as well as the “Forever Chemicals,” due to their persistency in the environment and humans, PFAS contain one or more carbon atoms, whose carbon-hydrogen bonds are replaced by carbon-fluorine bonds.
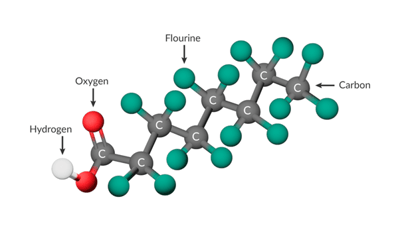
Image 1: Per- and polyfluoroalkyl substances – PFAS – are made up of a chain of carbon atoms, surrounded by fluorine atoms. The carbon-fluorine bond is one of the strongest in nature. Original 3D Image Source Wikipedia.
Carbon-fluorine bonds are the shortest and strongest chemical bonds observed in organic chemistry. The chemicals have high reactive stability and persistency. When multiple numbers (abbreviated as “n”) of fully fluorinated carbons are linked together, they are known as a “Cn” perfluorinated group, such as C6 and C8 PFAS. PFAS have many different structures, and the perfluorinated group gives them unique properties, which have been leveraged to the advantage of various commercial products and industrial processes.
First manufactured in the 1950s, PFAS were the main ingredients in non-stick and waterproofing treatments and coatings. The first firefighting foams with PFAS were created by 3M in the 1960s, and 3M was the sole supplier from the mid-1960s until 1973. The manufacture of PFAS increased in the late 1960s in the United States, especially for use in firefighting foams, bolstered by military applications following the deadly fire aboard a U.S. Navy aircraft carrier, the United States Secretary (USS) Forrestal, in 1967.
Now a major unintended consequence of the liquid form of this class of compounds is creating an environmental crisis. They are referred to as PFAS (per or polyfluorinated alkyl substances), and they have been shown to be a powerful endocrine disruptor (at a minimum). ¹
Why are PFAS desirable (and problematic)?
There are no known natural sources of PFAS, so their presence in the environment is solely anthropogenic. In the environment, the half-life of two commonly used PFAS – PFOS and PFOA – is 41 and 90 years, respectively. In humans the half-life of PFOS and PFOA ranges from 2.4 years to 21.7 years and 2.3 years to 3.8 years, respectively.
PFAS have excellent heat, chemical and thermal stability; stain, water and dirt repellence and wetting; and surfactant properties. Hence, they are used in a wide variety of products and processes, e.g., soil, water and oil repellent treatments for carpets and rugs; stain repellent treatments for textiles, leathers, and fabrics; in waxes, polishes, paints, adhesives, non-stick cookware, paper, and food packaging; pesticides; and industrial uses such as in firefighting foams, aviation hydraulic fluid, metal plating, electronic circuit board manufacture, and oil and gas production.
PFAS are a concern to the environment and human health due to their persistent, bioaccumulative, toxic (PBT) features, mutagenic and carcinogenic properties, and their adverse reproductive effects (mutagenic carcinogenic reproduction).
Sources of PFAS to the environment include direct manufacturing discharge to air and water, degradation, and/or precursors released from final commercial and consumer products and industrial uses, such as the contamination of soil, surface and groundwater. PFAS and precursors are also transported great distances in the environment via atmospheric and ocean currents, as evidenced by the detection of PFAS in the Arctic environment in both biota (e.g., polar bears, seals, etc.) and in soils and water. PFOS release to the environment involving water and soil comes from several sources, e.g., grease repellents for packaging, surface treatments for rugs and carpets, households (from vacuuming and cleaning of carpets and such waste to landfills), firefighting foams, and from incomplete combustion during incineration of PFOS-containing products, among other sources.
The oceans constitute the primary global reservoir for PFAS where concentrations of PFOA have been determined to be 10-80 pg/L (picograms per litre) in open waters. However, concentrations can be significantly higher near industrial areas, along the coast, or downstream from an airport. Long-range mobility occurs predominantly through oceanic currents for water soluble PFAS, but also through the atmosphere for more volatile varieties.
Further, PFAS adsorbs into soil and sediments, where it can be subsequently released into surface and ground waters and taken up by agricultural crops.
While acute toxicity of PFAS is low, a range of adverse effects have been observed in animal studies, such as effects on the liver, decreased thyroid hormone levels, effects on lipid metabolism, development of tumours in organs, and immunotoxicity and developmental toxicity. In humans, PFOS and PFOA have been associated with increased cholesterol levels, impact on infant birth weights, adverse effects on the immune system, increased risk for cancer and thyroid hormone disruption.
Exposure to PFOA and PFOS during pregnancy has been demonstrated to reduce foetal and post-natal growth, as well to increase infant mortality. This is especially alarming because these effects have been observed at low concentrations of PFOA and PFOS.
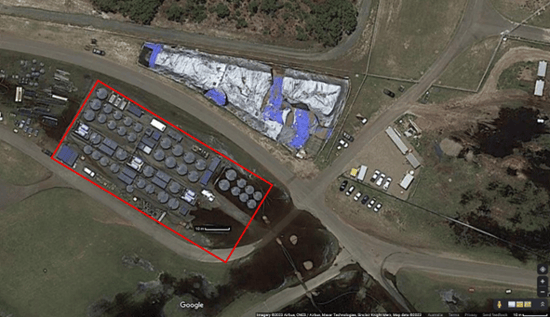
Image 2: Aerial shot of a conventional PFAS remediation at an Australian fire training facility. The visual shows the size of footprint, and thus difficulty, in treating water from such a facility and associated stormwater runoff with leaching PFAS. Photo: Google Maps
An international problem
In response to concerns regarding PFOS and PFOA, in 2009 the U.S. Environmental Protection Agency developed Provisional Health Advisories guidelines for drinking water and recommended maximum safe exposure concentrations for PFOS and PFOA at 70 ppt (parts per trillion). Australia quickly followed by implementing current PFOS healthy drinking water guideline to 70 ppt, however its PFOA limit is much lower at 560 ppt.
PFOS and PFOA are now restricted by EU REACH regulations and the United Nations’ Stockholm Convention on Persistent Organic Pollutants (POPs). Australia is one of many countries party to the convention, because of PFAS persistent, bio-accumulative and toxicity properties, as well as mutagenic, carcinogenic and reproduction (MCR) concerns.
Regarding elevated exposure of PFAS in the human body, the C8 Science Panel concluded that there were probable links and association of PFOS and PFOA to high cholesterol, thyroid disease, ulcerative colitis, pregnancy induced hypertension, and testicular and kidney cancers, as mentioned above.
For the above reasons, regulators, and policy makers, not only in Australia, but also in Canada, the U.K., the EU, and the U.S.A have taken measures to address PFAS-related environmental and human health issues and concerns.

Image 3: PFAS contamination in public waterways has been proven to cause health problems in humans and animals. Photo: iStock/michaelgeorgeau
Australian guidelines
In 2018, at the request of Environment Ministers around Australia, the Heads of EPAs Australia, and New Zealand (HEPA) and the then Australian Government Department of the Environment and Energy collaborated to develop a PFAS National Environmental Management Plan (NEMP), which was designed to achieve a clear, effective, coherent, and nationally consistent approach to the environmental regulation of PFAS. In the plan, several interim health and contamination guidelines were stipulated, such as health-based guidance values (including drinking water and health risks), soil criteria for human health and ecology, terrestrial and aquatic biota. NEMP version 3 is still under revision, and it is expected the revised guidelines are to be finalised sometime late in 2023.
Australia’s guidelines for complaint discharge has various categories dependant on the PFAS analyte and exposure scenario. NEMP 2.0 also has ecological protection risk values for various risk levels, e.g., 99% species protection value for PFOS at 0.0023 ug/L (micrograms per litre) and 19 ug/L for PFOA for high conservation value systems.
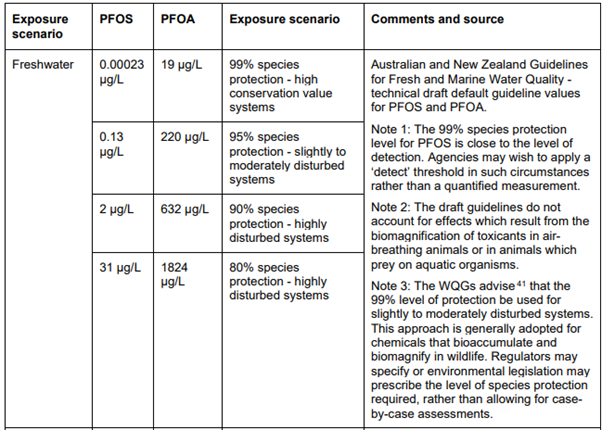
Table 1: Ecological water quality guideline values developed by water regulators
Methods and results
Current Treatment Methods
PFAS exhibit some specific characteristics that can in theory, be advantageous. By taking advantage of the chemical properties such as partial positive charges on carbon atoms along with partial negative charges on fluorine atoms, nucleophilic reactions offer promising potential. Hydroperoxide and superoxide radicals have been shown to destroy PFOA without detectable shorter-chain PFAS; however, the processes are challenged by the slow rate of reaction, which is typical of a nucleophile reaction.
The search for an in-situ approach is ongoing. Researchers have demonstrated that a new generation of hydroxyl radicals, as well as persulfate radicals in water, can be employed to destroy longer-chain PFAS. The problem is incomplete destruction leading to smaller, shorter-chain PFAS daughter products.
The onion analogy
The treatment of co-contaminants present in water is best approached by a multi-staged method to improve efficiency, durability (in the presence of spikes/upset conditions), operational costs and to extend service life . The staged filtration (removal) process is similar to the analogy of peeling the layers of an onion (refer Image 4). The first stage, or first layer peeled, is designed to remove the bulk of larger particulates, such as suspended solids, to improve the outcome of finer removal stages downstream.
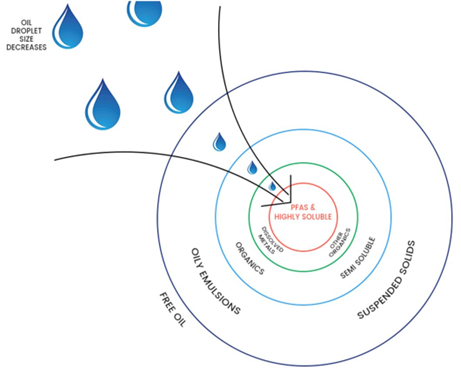
Image 4: OLEOLOGY’s “onion” analogy
Secondary stages should address the finer suspended particulates, phase-separated oil, and hydrocarbons (short and long chain) with final removal of other smaller size and soluble contaminants such as dissolved polychlorinated biphenyls (PCBs), emulsified hydrocarbons, organics & PFAS components, that are often found in wastewater, leachate, groundwater and fire training grounds.
The final ‘polishing’ stage, the onion core, is to create a high affinity to the remaining highly soluble PFAS compounds and precursors. By creating a homogenous mix prior to the final polishing stage, a higher efficiency and efficacy to achieve 99% species protection (or better) can be achieved on a regular basis.
Fouling of final polishing filtration media is commonly experienced in conventional (GAC/IX) wastewater filtration systems, due to the ineffectiveness of a staged approach for pre- and secondary treatments.
Sequential reduction of PFAS and co-contaminants has removed the industry “hurdle” of reduced service life of polishing media in conventional systems, resulting from either upset conditions or higher / untreatable concentrations of co-contaminants and emulsions in PFAS laden water.
Various PFAS removal and treatment technologies are now commercially available to remove and destroy PFAS, such as filtration using micro-, nano- and ultrafiltration, GAC, IX resins , absorbent (infused) media (MYX) and reverse osmosis (RO), amongst others. High thermal incineration, pyrolysis, and electrochemical oxidation can also destroy the C-F bonds of PFAS, to render them inert and safe.
PFAS contaminated water is commonly treated by using GAC and/or IX resins. Such a conventional approach is advantageous due to the initial low cost of deployment and has been a common method of water treatment for many decades.
GAC and IX media are commonly applied today for PFAS removal, however, were not designed for such specific use. As a result, the media is readily subjected to fouling due to the presence of co-contaminates such as emulsified oils, solids and metals, which greatly reduces efficiency. Drawbacks of such an approach include inefficiency (frequent replacement required), limited range (often requires combination with additional treatment options, such as reverse osmosis), and different sized PFAS chains varying with respect to water solubility (smaller chains are more water soluble, and therefore have a less affinity for binding agents).
The use of GAC in PFAS remediation is problematic because PFAS can be released in the presence of other organic compounds with a higher affinity, (steric hindrance). Due to the tendency of PFAS to form unusually weak attractions (i.e., van der Waals forces), they are easily displaced by other organic impurities which may be present. This results in a very unfavourable capture/release relationship, requiring the use of large amounts of media.
Another downside to GAC, IX and RO is the abundant generation of waste, both sludge and solid. Such solid waste is typically treated via combustion (it is important to ensure complete destruction, which often requires temperatures above 1200ºC). Sludge is a more difficult and costly waste item to transport and treat.
One newer treatment method by Foam Fractionation (FF) enables the removal of PFAS via the creation of a higher concentration within a bubble layer on the surface. The layer is then removed by vacuum to a more concentrated waste stream/s. Dependant on co-contaminates, further polishing can be achieved with additional treatment stages with a similar footprint, as outlined with GAC and IX.
Technology comparison
MyCelx Technology
MyCelx is a non-toxic, non-leaching, hydrophobic, absorbant of fixed chemistry, used for the removal of oil & organic (natural or synthetic) contaminants from fluid (water or air). Hence, there is no addition of chemical at site and there is no leaching of MyCelx chemistry into the water.
MyCelx technology has two main properties that sets it aside from other treatment methods;
1. MyCelx is hydrophobic (repels water) – producing a dry waste, easy to remove with no special tools or machinery.
2. MyCelx is oleophilic (affinity to hydrocarbons) - expanding beyond free and dispersed hydrocarbons and also includes chemically stable emulsified hydrocarbons, metals, organics and synthetic organics.
It is also notable that the footprint and scalability to treat larger volumes is not a linear progression, but almost exponential. The OLEOLOGY engineered system to treat PFAS only requires the operation of one pump, thus also reducing the power consumption, compared to other treatment options.
MyCelx vs Ion Exchange (IX) Resins
Regenerating absorbent media such as IX Resin, whilst attractive for increasing service life, has limitations. The concentrated PFAS waste brine solution must be contained and transported to a suitably qualified waste treatment site for PFAS. This waste is presented as liquid waste and has a high disposal cost to the end user. Additionally, in the presence of co-contaminants i.e., emulsified hydrocarbons, IX will experience early breakthrough, leading to more frequent replacement of media; thereby, adding to the operating costs and increasing personnel exposure to the waste.
MyCelx vs Granular Activated Carbon (GAC)
Carbon is commonly used as a water treatment method. It experiences mechanical limitations i.e., steric hindrance properties. Early breakthrough has been witnessed in the presence of co-contaminants while trying to attain ultratrace levels.
In a direct comparison using an equivalent media quantity with the same EBCT (Empty Bed Contact Time), GAC could not remove contaminants to the same efficiency as the MyCelx system.
A visual comparison between conventional treatment and MyCelx is displayed in Graph 1 and Graph 2: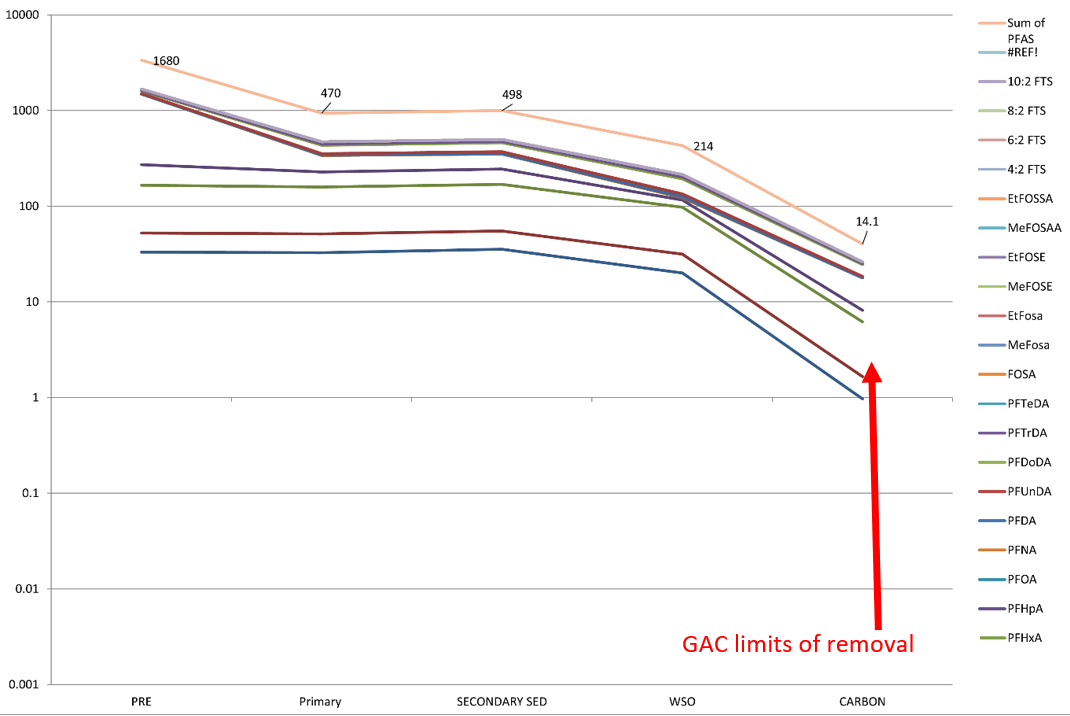
Graph 1: Displaying GAC removal quality for multiple PFAS analytes in sample water.
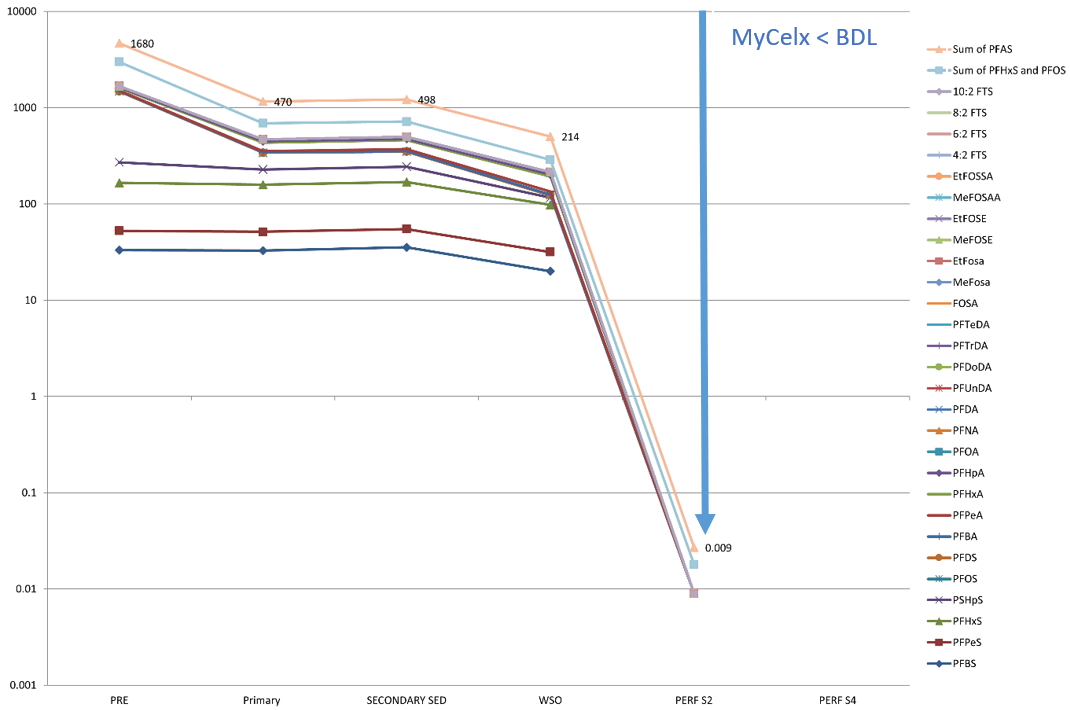
Graph 2: OLEOLOGY using MyCelx Performer media for same sample water, showing greater affinity for removal of PFAS analytes, compared against GAC.
Sites require a substantial quantity of GAC to achieve the same outcomes that MyCelx technology produces. As a result, the OLEOLOGY installation utilising MyCelx demonstrated a smaller footprint (by up to 10:1). The waste generated by a system with MyCelx filters is also up to 20 times less than that generated by a GAC system.
Conventional water treatment plants relying on the use of GAC and IX resin to remove PFAS, can only work efficiently for a short period of high co-contaminant load before breakthrough occurs. Residual hydrocarbon in groundwater is a common culprit and quickly overloads the GAC and IX. In turn, it saturates and breaks through the GAC and IX, leading to premature failure of the PFAS water polishing.
The Application of MyCelx Technology - Case Studies
Case Study 1 – Firefighting Foam Leaching (Oil and Gas Refinery)
Problem
Stormwater collected onsite in underground concrete sumps is contaminated with PFAS through firefighting foam leaching from the concrete.
Table 2 is the water analysis RAW results before treatment:

Table 2: Case Study 1 Stormwater Analysis Raw
Solution
To achieve the required quick turnaround, the OLEOLOGY PFAS Water Treatment System (PWTS) was delivered on skids, fully tested and ready for site connection, significantly reducing site installation and commissioning works.
The treatment solution included 6 skids:
Skid 1 – Pump, Controller and Monitoring Equipment
Skid 2 to 4 – Backwashable media to remove oily solids and protect the following MyCelx filtration stages. The sequence of filters removes dispersed & stable emulsions, along with soluble hydrocarbons to increase efficiency and to capture the various PFAS analytes.
Skid 5 & 6 – Further polishing of the water and highly soluble PFAS analytes. Two final Performer Polishing units were placed in series to mitigate the risk of breakthrough and achieve discharge to below detectable levels.
Result
Total PFAS was reduced to less than 0.0002 ug/L, which is less than ultratrace detection level and is compliant with the discharge requirements (see Table 3).
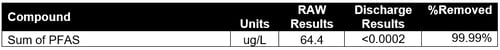
Table 3: Case Study 1 PFAS Removal Results
Case Study 2 – PFAS Leaching from Fire Training Ground (Airport)
Problem
Water onsite is contaminated with hydrocarbon and PFAS compounds through surface water collection on the concrete pad (after rainfall and training events). The results from sampling have shown that there are elevated PFAS contamination levels in the water runoff. The legacy contamination was causing a problem due to recordable levels and required a safe, compliannt discharge. The levels of PFAS required for discharge of treated water at site are: Sum of PFOS & PFHxS < 2 ug/L and PFOA < 10 ug/L (see Table 4).
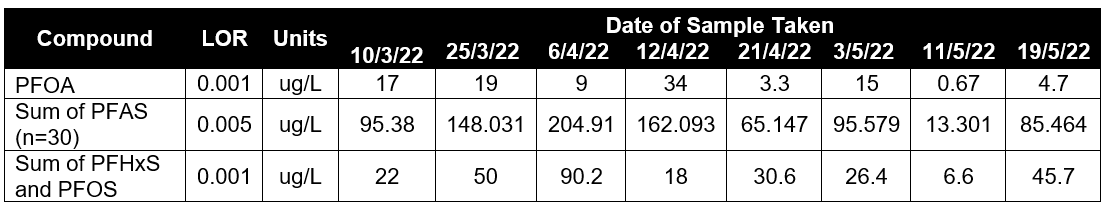
Table 4: Case Study 2 Raw Water Analysis Results
OLEOLOGY PFAS Water Treatment System stages:
The results are shown below in Table 5 below:
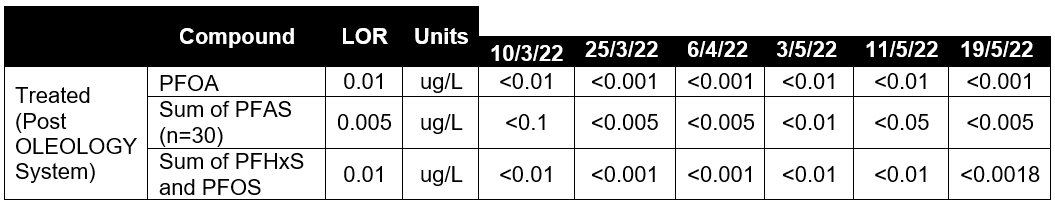
Discussions
MyCelx technology has shown to be unaffected by co-contaminants of hydrocarbons in numerous applications, thus the same system is designed to treat hydrocarbons and PFAS without adversely affecting efficiency of PFAS reduction - this is unique to the MyCelx prefiltration sequence.
The MyCelx system is designed to remove the PFAS compounds, organics, and hydrocarbons, instantly bonding them from the fluids to the media. With the technology matrix being a fixed chemistry within the cartridge filter and polishing media, there is no residual environmental liability resulting from the use of MyCelx.
MyCelx infused cartridges can capture up to 300 times their initial weight of contaminant mass. Highly soluble PFAS compounds, when polished through the MyCelx Performer Polishing media are reduced to below detectable levels (ultra-trace testing, Total Oxidisable Precursor Assay (TOPA) analysis).
PFAS is the sum of thousands of man-made compounds, many of them unknown to human beings because of the limitation of our testing technologies. With the advance of such technologies, more analytes of PFAS will be added to the current guidelines. Further studies on MyCelx technology performance, in terms of removing those new analytes, will be conducted. The PFAS Water Treatment System, infused with MyCelx technology, has proven successful in removal of PFAS, co-contaminants and precursors. Further advantages of the water treatment system have been identified for industry as lower cost, smaller footprint and better or broader performance treated water quality, compared to conventional technology.
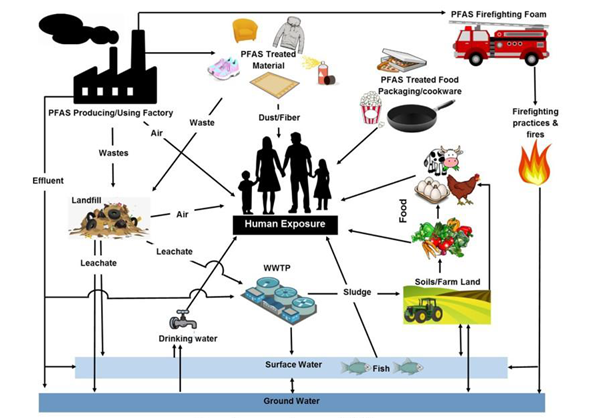
Image 5: Human exposure and sources of PFAS. Image: DWP adapted from Oliaei et all 2013.
Conclusions
Ideally, in any situation when treating PFAS impacted water, the system needs to be robust and can be best achieved in a multistage approach.
OLEOLOGY developed the PFAS water filtration system as a low-cost solution, with minimum waste, and small footprint to remove all PFAS analytes to below ultra-trace detection. The outcome is a testament to the engineering ingenuity within OLEOLOGY, as demonstrated with the MyCelx EPA accreditation of the resultant waste as “non leaching”, further improving the cost to treat by lowering waste disposal cost.
The removal of PFAS to Below Detectable Level (BDL) (ultratrace) and the broad affinity for removal of all PFAS analytes and precursors, confirms the superior performance of the OLEOLOGY water treatment systems incorporating MyCelx technology.
OLEOLOGY PWTS has not only treated water to below PFAS ultratrace levels, but it has also demonstrated successful removal of PFAS precursors. PFAS precursors, if not removed from the water, will potentially oxidise, and give rise to shorter chain PFAS analytes “reappearing”.
Over a successful period of operation with the OLEOLOGY PWTS in place, test results confirm that despite an inlet range of PFAS concentration from untreated raw water, the performance of the system is not affected by variations. Thus, continual compliance of final polished water is ensured to environment.
Acknowledgements
A special thank you to industry leader and verifier of OLEOLOGY work and performance review by Dr Jimmy Seow.
The Authors
Paul Callaghan is the Founder and Director of OLEOLOGY, a provider of engineered water solutions. His depth of knowledge results from a 33+ year career in the oil industry. For the past 12 years Paul has been working alongside International PFAS expert performance evaluation and analytical assessment of the MyCelx technology in the field.
Dr. Raymond Ooi is an experienced Chartered Professional engineer with Engineers Australia. He has over 18 years’ experience in the field of Process Engineering Design and Project Management with in-depth of technical knowledge and extensive R&D involvement with both academia and industry. Extensive working experience in Field Development, Design, Engineering and Operation.
Saasha Callaghan is an experienced professional in the wastewater treatment industry and has won numerous industry awards recognising her contribution in the Australian water industry. Saasha heads the business development and overviews project management for new system designs. She also oversees the company development activities, R&D initiatives, standards, and compliance requirements.
Lucas Callaghan is a water treatment professional with hands-on experience in building, testing, and conducting laboratory scale water testing. He has also demonstrated history of working in analysis and documentation of all internal and external water testing and analysis. Lucas has aided the developmental work within PFAS improvements.
References
1) Nisio, A et al. (2019) ‘Endocrine Disruption of Androgenic Activity by Perfluoroalkyl Substances: Clinical and Experimental Evidence’. The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 4, pp 1259-1271.
Endocrine Disruption of Androgenic Activity by Perfluoroalkyl Substances: Clinical and Experimental Evidence | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic (oup.com). Accessed Friday 24th February 2023
2) McGrath, M (2018) ‘Ozone hole mystery: China insulating chemical said to be source of rise’. BBC News, 9 July 2018. “FAQs Regarding PFAS Associated with AFFF Use at US Military Sites,” Report for ESTCP, Jennifer Field et al, 2017.
Harvard Style Bibliography | Format & Examples (scribbr.co.uk). Accessed Tuesday 10th January 2023.
3) DoH (2022) ‘Per and poly-fluoroalkyl substances’. Government of Western Australia Health Department. https://ww2.health.wa.gov.au/Articles/F_I/Guidance-Statements-on-Perfluorinated-Chemicals. Accessed Monday 9th January 2023.
